We’ve all experienced the discomfort of unexpected company. You get the phone call where someone lets you know they’ll be stopping by shortly. The rush begins to put the clutter in your house away in random cabinets and drawers. Then, you sweep the dust and dirt behind a dresser or under a rug. Your goal is to mask the disorder by making it look good temporarily. If you’re not careful, a process calibration of a pH measurement could be doing the same thing. You could just be forcing the measurement to look right temporarily. However, there are better practices to enable your measurement to be accurate for an extended range and period of time.
pH Measurement Slope and Zero Point
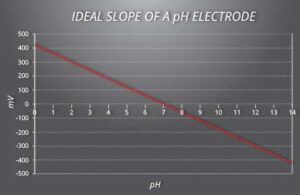
Before diving into the calibration process it’s important first to understand the slope and zero-point of a pH sensor. These values are specific to each sensor. The sensor’s slope is the linear correlation between the raw voltage reading and pH value. The theoretical slope value is 59.16 mV with the theoretical millivolt (mV) value at 7 pH being 0 mV (zero-point).
This means that in a perfect world, the mV value at 7 pH is 0 mV. It should then increase or decrease by 59.16 mV/per unit of pH as the value increases or decreases from 7 pH. Each sensor is unique, so the perfect world does not exist. The purpose of calibrating each sensor is to set the slope and zero-point relative to the theoretical values.
Process Calibration
A process calibration adjusts the offset error at a single point along the slope. It does not consider the effect on the slope across the entire scale. This offset might help you read more accurately at that single point, but as the pH value increases or decreases, you’ve introduced an additional offset that will cause inaccurate pH readings across the rest of the scale. A process calibration is not recommended for accurate measurements across a wide pH range.
There are some scenarios where a process calibration is an accepted practice.
- The pH reading in your process stays constant.
If you have an application where you’re maintaining pH at one set point, then there’s no need to worry about the rest of the scale. You can perform a process calibration at the pH value you wish to maintain. You won’t need to worry about the additional offset that’s introduced across the rest of the scale. - The sensor has a fixed slope over the entire measurement range.
In this case, there’s no need to introduce a second calibration point. A process calibration is an acceptable practice to eliminate the measurement offset.
Two Point Calibration
A two point calibration is more precise than a process calibration. In doing this, we adjust the sensor offset at two different mV values, creating accurate measurements across the entire pH scale. It is typically recommended that one of the two points used for calibration is 7 pH (0 mV). This ensures we accurately set the zero point of that sensor.
After a two point calibration, the sensor or transmitter provides a new slope and zero-point. They display the slope as a percentage of the theoretical value of 59.16 mV, with 100% indicating exactly 59.16 mV. A good calibration has a zero point as close to 0 mV, and a slope as close to 100% as possible.
When working with pH sensors, don’t sweep your calibrations under the rug. Doing a process calibration may make your measurement look good temporarily but you will be masking inaccuracies. It’s our recommendation to perform a two point calibration to guarantee the most accurate measurement across the entire scale and ensure you get a good “clean” pH measurement for all future company.
