Oxygen sensors are essential to various industrial applications for quality monitoring, process control, and environmental compliance. Oxygen sensors analyze levels of free oxygen in liquids and gases. For liquid analysis applications, specifically wastewater treatment, microorganisms are crucial to breaking down harmful compounds. Typically, such organisms require a minimum of 5 ppm (parts per million) of dissolved oxygen to survive. Knowing these levels are crucial to the health of such processes. In many gas-based applications, oxygen levels are extremely important to analyze, particularly in applications that utilize nitrogen blanketing. In such applications, operators must ensure oxygen levels are kept to a minimum to avoid potentially catastrophic reactions.
Many factors influence oxygen levels, including temperature, water depth, salinity, bioactivity, and atmospheric pressure. Understanding these variables is important to accurately monitor oxygen in both gaseous and dissolved forms. Let’s explore how oxygen sensors work.
HOW OXYGEN SENSORS OPERATE
There are two primary styles of technology for oxygen sensors: Electrochemical (Clark-Style/Amperiometric) and Optical (Luminescent). Traits that are unique to either sensor type make them applicable to certain conditions in various applications. We will examine the methods by which each style of sensor operates so that you can determine which technology best suits your application.
ELECTROCHEMICAL SENSORS
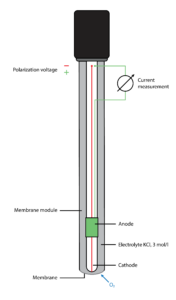 Electrochemical sensors are capable of interpreting higher ranges of oxygen values than luminescent sensors, measuring up to 45 ppm. This makes them more appropriate for heavier applications such as chemical and water treatment.
Electrochemical sensors are capable of interpreting higher ranges of oxygen values than luminescent sensors, measuring up to 45 ppm. This makes them more appropriate for heavier applications such as chemical and water treatment.
These sensors are equipped with an oxygen-permeable membrane attached to an anode and a cathode that are immersed in a reference electrolyte. To accurately interpret the voltage, the anode, and cathode must be polarized through a transmitter or another power source. The cathode is typically constructed of a precious metal that stabilizes the polarization of the sensor. When measuring in-process, the oxygen passes through the membrane. Once through, a chemical reaction in the electrolyte produces a voltage registered by the anode and cathode. The higher the oxygen levels are, the higher the generated current will be. A transmitter will then interpret the voltage into a readable oxygen value.
OPTICAL SENSORS
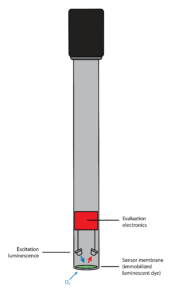 Optical oxygen sensors are a relatively new style of sensor on the market. Advancements in this form of technology have led to a growing presence of these sensors in various industrial processes.
Optical oxygen sensors are a relatively new style of sensor on the market. Advancements in this form of technology have led to a growing presence of these sensors in various industrial processes.
They operate using an internal LED light and an external cap that contains an oxygen-sensitive fluorescent dye layer. Additionally, an internal receiver works in unison to translate light waves into readable oxygen values. Without the presence of oxygen, the wavelengths generated between the light and the dye layer will move rapidly to the transmitter. Once oxygen comes in contact with the dye layer, the waves will move much slower.
MAINTENANCE IS KEY TO KEEPING SENSORS FUNCTIONAL & ACCURATE
The maintenance techniques required to prolong sensor life and ensure accurate measurements are also unique to the model of the sensor.
Electrochemical Maintenance: Electrochemical sensors require a bit more maintenance than their Luminescent counterparts. As previously mentioned, users must polarize the reference system through a transmitter or another power source. It can take several hours to stabilize the reference system. Additionally, the anode and cathode must be cleaned regularly to avoid wear, particularly on the precious metal cathode. Readings will begin to drift if the reference system is not properly cared for, and measurements will be inaccurate.
Optical Maintenance: The most important aspect of maintaining a luminescent oxygen sensor is the condition of the external cap containing the dye layer. If kept in a corrosive or otherwise abrasive environment for too long, the fluorescent dye layer will begin to break down. A damaged dye layer will cause the wavelength patterns of the LED light to change, affecting the accuracy of the measurement. However, damaged dye layer caps are easily replaceable without requiring an entirely new sensor.
DISCOVER WHICH MODEL WORKS FOR YOU
Both models of oxygen sensors have strengths that provide them with a role to play in various applications requiring oxygen measurements. Find which model works best for your process by exploring our oxygen sensor options.


