It is commonplace when calibrating pH sensors to use buffer solutions with values that bracket the nominal measuring range of your application. For example, a measurement range of 5 to 6 pH is best suited for the use of buffers 4 and 7 when doing a two-point calibration. However, not all pH measurements are acidic, and there are applications where two-point or three-point calibrations require the use of high pH (alkaline) buffers. Here we will review the volatile nature of these buffers and the recommendations for their proper use in order to produce accurate high pH measurements.
Buffers are aqueous solutions that maintain pH stability despite small additions of acids or bases. This stability is useful when technicians calibrate loops in the field where sensor cleanliness could be questionable. While acidic and neutral buffers are particularly stable, alkaline buffers absorb carbon dioxide from the air. The absorbed carbon dioxide reacts with water in the buffer solution to form carbonic acid; thus decreasing the pH value of the solution. So, as soon as you expose a high pH value buffer to air, it begins to neutralize.
Experimenting with Alkaline Buffers
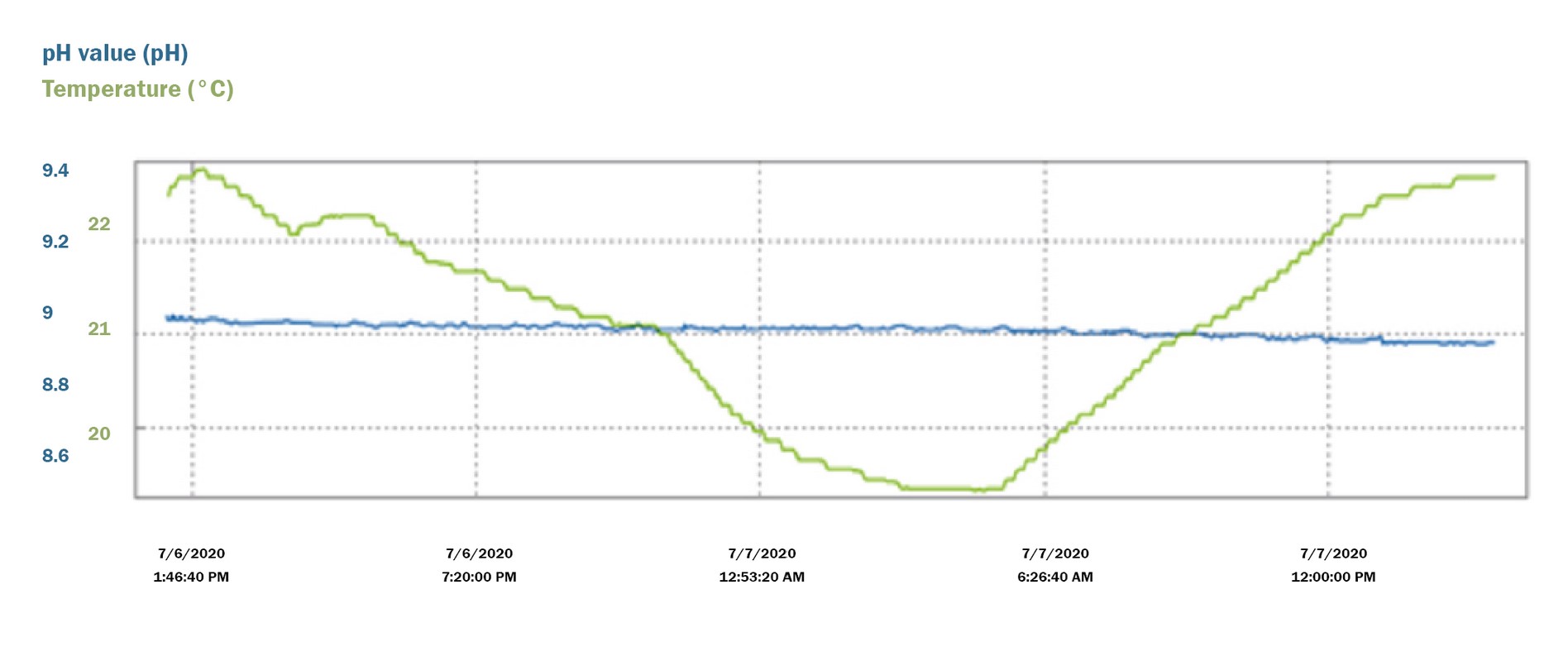
The data below trends the carbonic acid effect on 50-mL of a 10.01 pH NIST technical buffer measured in an open 150-mL beaker over 24-hours. The pH of this buffer effectively declined by 0.15 pH from 10.04 to 9.89 during this period.
The sensor used for this measurement received a two-point calibration before the experiment. We used fresh buffers 7.00 and 10.01 (pH @ 25° C) from the same buffer set. Note the zero and slope:

We calibrated the same sensor after the experiment with fresh buffer 7.00 from the same set along with the degraded buffer 10.01. Note the zero and slope of this calibration in contrast to the above:

While the zero maintained stability within 0.006 pH, the slope degraded from 58.7 mV/pH (99.2%) to 55.9 mV/pH (94.5%). Now imagine a technician using degraded alkaline buffers for calibrating loops in an environmental discharge or a critical control point. This would lead to measurement accuracy issues at the higher end of the range.
Best Practices for Calibration Accuracy
In a previous article, we discussed pH calibrations using automatic calibration functionality. The following is a list of recommendations to prevent measurement errors when using alkaline buffers:
- Use separate containers for calibration. Never immerse a sensor into a buffer within its storage container.
- Fully use opened containers or discard them within a few days of opening. Mark any container with the date of opening to keep track of elapsed time.
- Close containers after dispensing to prevent contamination by air and/or dust.
- Use the smallest container size possible. Consider single-dose packets if use is infrequent. Consider dispensers that prevent air from entering the container if use is frequent.
- Emerging digital technologies facilitate offline calibrations and sensor maintenance. Calibrations conducted within climate-controlled environments reduce the risk of contaminating buffers.
- We recommend using buffers manufactured by the National Institute of Standards & Technology (NIST) Standard Reference Material (SRM), or DIN 19266.
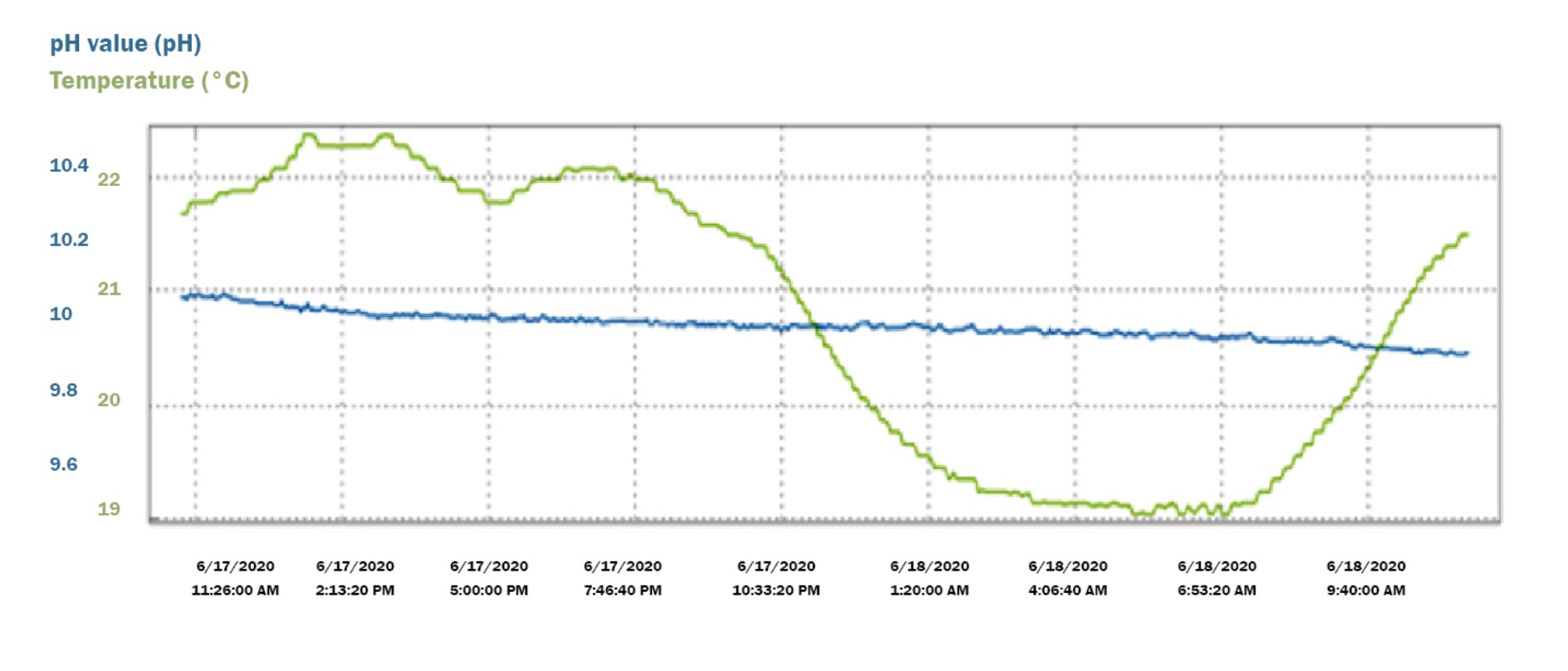
Technical buffers that are NIST traceable, or DIN 19267 are also suitable for most applications. Pay attention to buffer values with respect to the accuracy requirements of the application. - Buffers 9.00 (@ 20° C) or 9.18 (@ 25° C) can often satisfy the requirement for a 2nd or 3rd calibration point. Buffers in these ranges offer greater stability than those ≥10.00 pH. The data below illustrates this stability in contrast to the 10.01 buffer previously mentioned. The pH of this buffer 9.00 also declined over 24-hrs, but only 0.06 pH from 8.97 to 8.91:

- Complete a two-point calibration using one acidic and one neutral buffer (~4 and ~7) if the condition of your alkaline buffer is in question. Modern combination pH sensors have reliable linearity. You can expect greater accuracy in the alkaline range when using good buffer 4 and 7 than if using a good buffer 7 and bad buffer 10.
Summary
There are many challenging applications and potentially costly events associated with measuring pH. Measurements in alkaline ranges and high temperatures are notorious for damaging sensors. Additional costly events include environmental discharge being outside of allowable limits and deviations in critical control points that produce off-spec products. Measurement deviations between grab samples and field instruments create angst between operations and maintenance personnel.
Calibrations are like any equation: garbage-in equals garbage-out. The premature disposal of sensors and the more costly effects of an inaccurate measurement is often caused by improper calibrations. Use accurate and fresh buffer solutions for every calibration to ensure you minimize the cost of ownership.